Guide to Central Venous Catheter Securement and Stabilization Devices
All central vascular access devices should be stabilized to prevent complications such as device movement or pistoning in the vessel that may cause phlebitis, cause the introduction of microorganisms into the vessel, and/or unintentional loss of venous access. The methods used to stabilize a device should not interfere with the assessment or monitoring of the site and should not impede vascular circulation or delivery of the prescribed therapy. Vascular securement can be grouped into 3 primary categories: sutures/staples, subcutaneous anchoring device, and cutaneous/adhesive based securement devices. Below is an overview of the securement and stabilization devices available on the market. You may click on the product links, where available, to learn more about the manufacturer and device.
Guide to Central Venous Catheter Securement
and Stabilization Devices
| Sutures and Staples | |
| Photo | Product |
 |
Stapled CVC |
 |
Sutured CVC |
|
Subcutaneous Securement Device |
|
| Photo | Product |
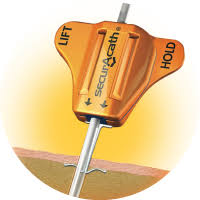 |
SecurAcath® |
|
Cutaneous/Adhesive Based Securement Devices |
|
|
Photo |
Product |
 |
Centurion WingGuard® |
 |
StatLock™ PICC Plus |
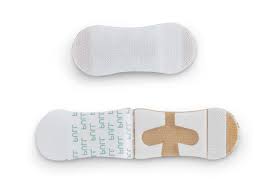 |
Grip-Lok® |
 |
Centurion HubGuard Securement Device |
 |
3M™ PICC/CVC Securement Dressing |
 |
Centurion CVC Securement Anchor |
 |
Braun Clik-FIX® |
 |
The Bedal 2 PICC / CVC |
For reference and educational use only. Pedagogy does not endorse or recommend the use of any product or product manufacturer.
Are you a manufacturer of a Central Venous Catheter Securement and Stabilization device that isn't listed here? Please contact us at support@pedagogyeducation.com and we would be more than happy to list your device as well!
Are you a manufacturer of a Central Venous Catheter Securement and Stabilization device that isn't listed here? Please contact us at support@pedagogyeducation.com and we would be more than happy to list your device as well!